

We already mentioned temperature and time being key factors for successful recrystallization. It is very easy to get a precipitate, but very difficult to get crystals. Crystals, however, are often composed solely of one compound. A precipitate may not pure, because it can contain several compounds. This can happen for a variety of reasons, but a student may have taken the very hot solution and placed it directly on a cold surface to cool (a process called “shock cooling”) or even plunged the hot solution into an ice bath. A precipitate is simply a mixture of compounds in the solution that crash out. As the solubility decreases, the solution at some point becomes supersaturated and crystals will start to form.īefore we move on, let me address the main problem associated with crystallization: the formation of precipitate, versus crystals. As the temperature starts to decrease, so does the solubility of the compound. Because most solids have a better solubility at higher temperatures, we can sat- urate or almost saturate a solution at high temperature (usually the boiling temperature of the solvent), and then slowly allow the solution to reach room temperature.
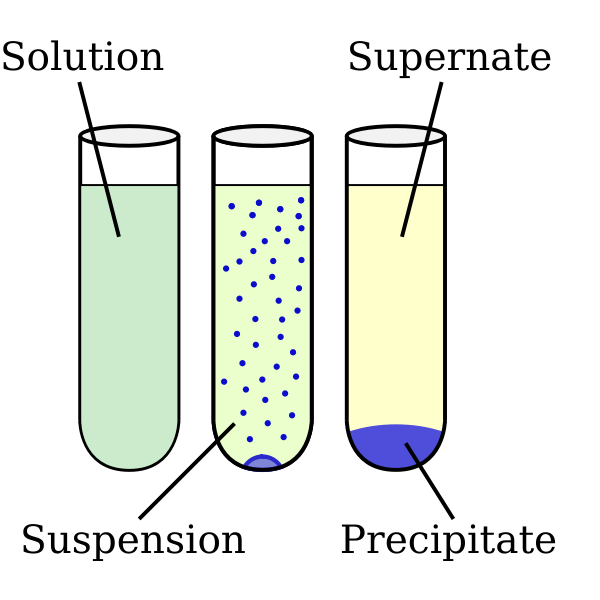
The key features necessary for a successful recrystallization process, are a very controlled temperature decrease and sufficient time. Recrystallization is therefore a purification technique. The goal, is to obtain a compound in high purity as uniform crystals. Sounds easy, doesn’t it? It is actually a very challenging process to get completely right. The key features of this technique is causing a solid to go into solution, and then gradually allowing the dissolved solid to crystallize. Recrystallization is a laboratory technique for purifying solids.


 0 kommentar(er)
0 kommentar(er)
